Often termed the “hidden hunger”, subclinical micronutrient deficiencies have no immediate physical signs and are insidious in causing diseases in children.1 Realizing the need for increased awareness around micronutrient deficiency as a public health problem, the Child Nutrition Advisory Group (CNAG) recently hosted its 2nd Expert Workshop in March 2014. Chaired by Dr Henry Au Yeung and featuring key presentations by Professor Andrew Prentice from the UK and Dr Sophie Leung from Hong Kong, the workshop provided a platform for a select group of local paediatric experts to share, discuss and address pertinent issues pertaining to the importance of micronutrients in modulating growth and immunity in children.
Nutritional modulation of immunity and growth: Role of micronutrients in children
Professor Andrew Prentice
Professor of International Nutrition
London School of Hygiene and Tropical Medicine
Head, Medical Research Council International Nutrition Group
MRC Unit, The Gambia
There is an apparent relationship between gross domestic product per capita growth and early childhood undernutrition, alluding to economic growth being a necessary condition for nutritional improvement.2 Even so, a robust economy does not guarantee improved child nutrition. Level of public health and education expenditures are also significant determinants of changes in nutrition status in children.3 Drawing from his research experience in developing countries, Professor Prentice summarized recent findings on the roles of micronutrients and provided insights into how key learnings may be translated and applied to the current nutrition landscape of Hong Kong.
Breastfeeding and importance of adequate early nutrition
Breastfeeding remains the gold standard for infant feeding and should be exclusive until 6 months of age. Data from low-income countries has shown that non-breastfed infants had a higher risk of dying compared with those who had been predominantly or partially breastfed.4 Comparison of child growth patterns in low- and lower-middle income countries showed that growth faltering and stunting in early childhood are pronounced, confirming the need for optimal nutrition during the critical periods, which are during pregnancy and the first 2 years of a child’s life, as well as the preconception period.5,6
There is a need for optimal nutrition during the critical periods, which are during pregnancy and the first 2 years of a child’s life, as well as the preconception period
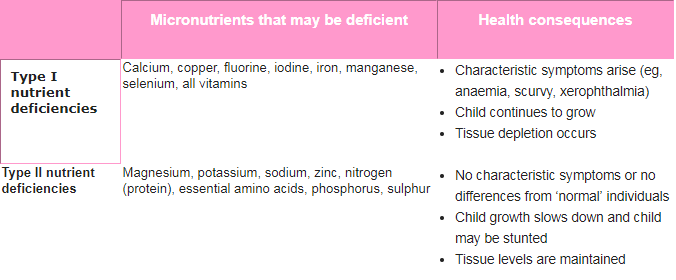
Type I and II nutrient deficiencies and growth
The result of undernutrition, either overall growth failure or specific micronutrient deficiencies, depends on the actual nutrient that is deficient in the body. Nutrients are classified as either type I or type II nutrients, based on the effect a deficiency has on the body (Table).7
Table. Specific nutrient deficiencies and their consequences7
Nutrition–immunity interactions
Nutrient insufficiency or imbalance has interactive effects on immunocompetence.8 The challenge lies in maintaining stability of the triangle formed by the interaction between micronutrient, infection and immunity during critical periods.
Vitamin D
Adjunctive vitamin D therapy has been shown to reduce the incidence of respiratory tract infections, especially in the paediatric population.9 Vitamin D receptors (VDRs) are found on most immune cells,10 and several genetic variations have been identified in the VDR. These polymorphisms may impact a child’s vitamin D absorption and disease susceptibility.11 More concrete evidence is required to elucidate the role of VDR polymorphism in immunity and bone biology.
Selenium
Selenium is an important mediator for protection against oxidative stress. Recent data has shown that selenium deficiency results in increased viral pathogenicity and altered immune responses. This deficiency results in specific viral mutations, changing benign viruses into virulent strains,12 which might affect the virulent H5N1 avian influenza virus.13
Zinc
Zinc deficiency can lead to decreased immunity and an increased risk of infection.14 Preventive zinc supplementation in healthy children can reduce mortality due to common causes like diarrhoea and pneumonia, as well as improve linear growth.15,16 Moreover, zinc supplementation of 10–40 mg/day has also been shown to effectively reduce the duration and severity of diarrhoea, diarrhoea hospitalization rates and diarrhoea mortality in children aged 5 years or younger.17
Iron
Iron is essential for both host and pathogen, and complex systems of acquisition and utilization have evolved as a result of competition for iron.18 While iron deficiency is known to decrease immune function, some investigators have hypothesized that this deficiency actually protects against certain infectious diseases, and it has been shown that iron supplementation can increase the risk for infectious diseases, such as malaria in children in endemic areas.19
Long-chain polyunsaturated fatty acids (LC-PUFAs)
LC-PUFAs are potentially potent anti-inflammatory agents at sufficiently high intakes and may be of therapeutic use in a variety of acute and chronic inflammatory settings. Evidence of their clinical efficacy is stronger in some diseases (eg, rheumatoid arthritis) than in others (eg, asthma in the paediatric population and Crohn’s disease). As such, better-designed and larger trials are required to assess the therapeutic potential of LC-PUFAs in inflammatory diseases.20
Summary
While more conclusive clinical trial evidence, as well as more reliable tools to assess nutritional status in children, are warranted, Professor Prentice recommended employing a “life course” perspective to health promotion and disease prevention to address disparities in maternal and child nutrition. Besides continuing efforts to promote breastfeeding, timely nutrition, diet and lifestyle advice should be given to prevent and overcome micronutrient deficiencies in Hong Kong children.
Unmasking micronutrient deficiencies in Hong Kong children
Dr Sophie Leung
Honorary Associate Professor in Paediatrics
The Chinese University of Hong Kong
Hong Kong
In Hong Kong, nutritional problems in children are related to overnutrition (excess protein and caloric intake) and unbalanced diet rather than undernutrition, and, as such, micronutrient insufficiencies and deficiencies are commonly overlooked. “The modern diet, comprising mainly of refined carbohydrates and saturated fats with low levels of micronutrients, such as antioxidants, phytochemicals, carotenoids, and trace minerals, has contributed to compromised immune function development in Hong Kong children,” commented Dr Leung.
During infancy and early childhood, the requirement for micronutrients is high relative to body size to meet the need for rapid growth and development. Five case studies, involving children with chronic constipation, eczema or hair loss, were presented to show how well-designed nutritional interventions, consisting largely of whole grains, vegetables and fruits, can successfully impact micronutrient intakes and nutritional status outcomes in children. A simple questionnaire on diet history, frequency of milk intake, and the quality and quantity of solid food and snack intake should be considered as part of routine healthcare for children.
One of the more interesting observations from the cases presented relates to the growth pattern of Hong Kong infants. Overnutrition in mothers during pregnancy may be associated with excessive weight gain in the foetus, as well as in infants during the first few months of life.21 As such, self regulation, mapped by a physiological downward crossing of weight percentiles, may take place between 4 and 10 months of age in order to achieve the inherited growth percentile by 1 year old. Dr Leung pointed out that if this period of slow weight gain was misinterpreted as failure to thrive, dietary intervention in the form of introducing high-protein and high-calorie foods may lead to further decrease in intake of micronutrient rich, natural plant-based foods. It must be noted that the weight of infants should always be interpreted together with length of infant and parental size.22
Expert recommendations
To address micronutrient deficiencies, public health policies must be generated to discourage the consumption of high-calorie, high-protein and low-nutrient foods and encourage the consumption of natural, plant-based whole foods. Parents and children need to be imparted the knowledge regarding a healthy, well-balanced diet (comprising more whole grains, pulses, fruits and vegetables) so as to make informed food choices. “Most importantly, parents should be educated that the growth needs of the child determine his or her energy needs, and that children should not be encouraged to eat in excess,” concluded Dr Leung.
There is a need for optimal nutrition during the critical periods, which are during pregnancy and the first 2 years of a child’s life, as well as the preconception period
Expert insights: Q & A
Q: Taking into account the host–pathogen battle for iron hypothesis, is it, therefore, not advisable for children to consume iron-rich foods or iron-fortified formula milk?
Professor Prentice: The hormone hepcidin plays a role in regulating iron metabolism, integrating signals from iron deficiency or surplus and during infections. As such, parents should not be giving large, non-physiological doses of iron, as these can overwhelm the natural processes of chaperoning iron around the body. The amount of iron consumed should remain within the normal food matrix, even with the case of iron-fortified formula milk, although this has yet to be formally tested.
Q: Serum zinc levels in atopic children in Hong Kong is relatively low. Even though zinc can be found in red meat and seafood, these foods are typically avoided by these children. What are your comments on this?
Professor Prentice: Practitioners must consider the possibility of alterations in acute phase proteins when interpreting zinc laboratory data. Decreases in serum zinc have been described in association with the acute phase response, making these levels difficult to interpret. Nonetheless, avoiding zinc-rich foods in atopic dermatitis is a misguided approach as zinc is an anti-inflammatory mineral.
Q: Animal products are rich in riboflavin (or vitamin B2) and essential amino acids. Yet, some physicians have advocated keeping meat and animal product consumption at a minimum in children. What is their rationale for doing so?
Dr Leung: Riboflavin or vitamin B2 can also be found in green leafy vegetables and broccoli. The full spectrum of essential amino acids can be obtained by consuming one type of animal product or through a variety of plant-based foods. Recent research has also shown that plants contain phytochemicals, which provide plants with colour and have beneficial effects in humans. In general, Hong Kong children already have a high consumption of animal products. As such, further nutrition education should focus on balancing animal and plant sources of proteins and encouraging adequate consumption of vegetables and fruits.
References
1. Food and Agriculture Organization. The scourge of “hidden hunger”: global dimensions of micronutrient deficiencies. Available at: ftp://ftp.fao.org/docrep/fao/005/y8346m/y8346m01.pdf. Accessed 18 April 2014.
2. United Nations Development Programme. Economic Growth and Child Undernutrition in Africa. Available at: http://www.undp.org/content/dam/rba/docs/Working%20Papers/Economic%20Growth%20and%20Child%20Undernutrition. pdf. Accessed 2 June 2014.
3. Gillespie S, Mason J, Martorell R. UN ACC/SCN State-of-the-Art Series Nutrition Policy Discussion Paper No. 15. 1996. Geneva: United Nations, Administrative Committtee on Coordination/Subcommittee on Nutrition.
4. Bahl R, et al. Bull World Health Organ 2005;83:418-426.
5. Prentice AM, et al. Am J Clin Nutr 2013;97:911-918.
6. Victora CG, et al. Pediatrics 2010;125:e473-e480.
7. Golden MH. SCN News 1995;10-14.
8. Kubena KS, McMurray DN. J Am Diet Assoc 1996;96:1156-1164; quiz 1165-1156.
9. Charan J, et al. J Pharmacol Pharmacother 2012;3:300-303.
10. Wang Y, et al. Arch Biochem Biophys 2012;523:123-133.
11. Valdivielso JM, Fernandez E. Clin Chim Acta 2006;371:1-12.
12. Beck MA. J Nutr 2007;137:1338-1340.
13. Harthill M. Biol Trace Elem Res 2011;143:1325-1336.
14. Fischer Walker C, Black RE. Annu Rev Nutr 2004;24:255-275.
15. Yakoob MY, et al. BMC Public Health 2011;11(Suppl 3):S23.
16. Imdad A, Bhutta ZA. BMC Public Health 2011;11(Suppl 3):S22.
17. Walker CL, Black RE. Int J Epidemiol 2010;39(Suppl 1):i63-i69.
18. Doherty CP. J Nutr 2007;137:1341-1344.
19. Caulfield LE, et al. Am J Trop Med Hyg 2004;71:55-63.
20. Calder PC. Am J Clin Nutr 2006;83:1505S-1519S.
21. Viswanathan M, et al. Evid Rep Technol Assess (Full Rep) 2008;(168):1-223.
22. Leung SS. The Hong Kong Medical Diary 2010;15:4-7.
