The Child Nutrition Advisory Group (CNAG) hosted an Expert Workshop in August 2013. This forum provided a platform for a select group of Hong Kong healthcare professionals to share, discuss and address pertinent issues regarding the role of long-chain polyunsaturated fatty acids (LC-PUFAs) in paediatric neurobehavioural development. Chaired by Dr Henry Au-Yeung, this workshop also featured key presentations by Professor Susan E. Carlson from the University of Kansas, USA and Dr Sylvia Doo from St Paul’s Hospital, Hong Kong.
LC-PUFAs: Their roles in neuro-behavioural development and outcomes
Professor Susan E. Carlson
AJ Rice Professor of Nutrition
University of Kansas Medical Center, USA
Long chain polyunsaturated fatty acids (LC-PUFAs) include omega-6 and omega-3 fatty acids. In the developing brain, arachidonic acid (AA) and docosahexanoic acid (DHA) are the two most abundant LC-PUFAs.1 Professor Carlson explained that DHA, a major structural component of the brain grey matter, promotes neuronal contact and intercellular communication which are both important functions for memory. DHA accumulation begins in utero and increases exponentially up to at least 2 years of age.2
Breastfeeding and importance of adequate early nutrition
Effects of pre-natal DHA levels
Behavioural and cardiac responses are linked with the distribution of time infants spend in different phases of attention.3 “Shorter look duration in early infancy (at about 4 months) has been found to be associated with higher performance in childhood. After the age of 9 months, the converse is true,” explained Professor Carlson.
Colombo et al’s longitudinal study found that infants born to mothers with a high DHA at birth had an accelerated decline in looking in the 1st year, longer examination during single-object exploration and less distractibility in the 2nd year. These findings underscore evidence suggesting a link between DHA and cognitive development in infancy.4
Maternal DHA supplementation also confers improvements in infant clinical outcomes. In the Kansas University DHA Outcome Study (KUDOS), women who received DHA supplementation (mean dose of 469 mg/day) had higher maternal serum and cord RBC-phospholipid-DHA (p<0.001) than those in the placebo group (α-linoleic acid). Infants born to DHA-supplemented mothers also had longer gestation duration (p=0.041), fewer early preterm births (<34 weeks of gestation) (p=0.025) and shorter hospital stays for infants born preterm (p=0.026).5
Effects of DHA supplementation on infants, toddlers and children
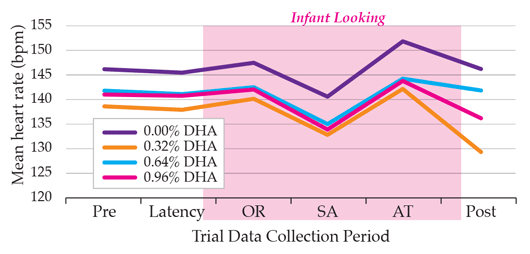
The DHA Intake and Measurement of Neural Development (DIAMOND) trial was conducted to determine whether DHA supplementation had a dose-dependent impact on infant cognitive function. Four different levels of DHA supplementation (at 0.00, 0.32, 0.64, and 0.96% of total fatty acids) were tested when infants were at 4, 6, and 9 months of age.3 As the autonomic nervous system (ANS) regulates both cognitive and vital functions, such as heart rate, this study measured the heart rate of infants while they were visually engaged. Using this approach, it was found that even at the lowest dose of DHA supplementation (0.32%), infants had lower heart rates than those unsupplemented,3 with lower heart rate as an indication of higher ANS function as explained by Professor Carlson. (Figure 1 below shows heart rate at 6 months). Additionally, infants whose diet was supplemented with either 0.32% or 0.64% of DHA spent proportionately more time engaged in active stimulus processing (sustained attention) than infants fed the unsupplemented formula.3 The increase in sustained attention is an indication of engaged cognitive processing and is seen as a positive benefit of supplementation.3
Systematic reviews and meta-analyses of studies that employed a global test of development, the Bayley Scales of Infant Development (BSID) to measure cognitive function of infants at 18 months have consistently shown no benefit of LC-PUFA supplementation.6 However, when testing was extended up to 6 years and more targeted tests were employed, long term cognitive benefits of DHA supplementation in infancy were found. Children between the ages of 3 to 5 whose diet was supplemented with AA and DHA for the first 12 months of infancy performed better on rule-learning and inhibition tasks than their control counterparts.6 At age 5, these children also performed better on a test of receptive language, the Peabody Picture Vocabulary Tests, and at age 6 had higher verbal and total IQ on the Weschler Primary Preschool Scales of Intelligence. Results from this study suggest the importance of using appropriate tests that measure age-specific rather than global cognitive function when assessing the impact of DHA supplementation in children.
DHA supplementation in older children can also be beneficial. In the DHA Oxford Learning And Behaviour (DOLAB) study, 7–9 year old children (initially underperforming readers) whose diet was supplemented with DHA (600 mg/day) exhibited significant improvements in reading (p<0.04). Active treatment significantly reduced parent-rated ADHD-type symptoms, although little or no effects were seen for teacher-rated behaviour or working memory.7
Paediatric neurobehavioural disorders in Hong Kong – prevalence and clinical management
Dr Sylvia Doo
Head, Child Development and Assessment Centre
St. Paul’s Hospital, Hong Kong
Prevalence and trends of neurobehavioral disorders in Hong Kong
Although attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD) are classified as two distinct neurobehavioral disorders, symptomatically, there is a great deal of overlap.8
Dr Doo noted that ADHD is characterized by inappropriate attention, hyperactivity and impulsivity. “The three most common co-occurring conditions are dyslexia, specific language impairment and developmental coordination disorder,” she added. Worldwide pooled prevalence of ADHD is 5.29%.9 In Hong Kong, previous data indicated that 3.9% of early adolescents and 6.1% of Primary 1 schoolboys have been diagnosed with ADHD.10, 11
“Autism spectrum disorder (ASD) is a behavioral syndrome characterized by impairment in social interaction as well as slow development of language and communication skills. They may also exhibit stereotypic and inflexible behavior and display a narrow interest pattern and/or motor mannerism. These symptoms typically occur before the age of 3 years,” explained Dr Doo. According to figures by Child Assessment Service, Department of Health, from 1997 to 2003, the number of newly diagnosed cases of ASD in Hong Kong had tripled.12
Management of neurobehavioural disorders in children
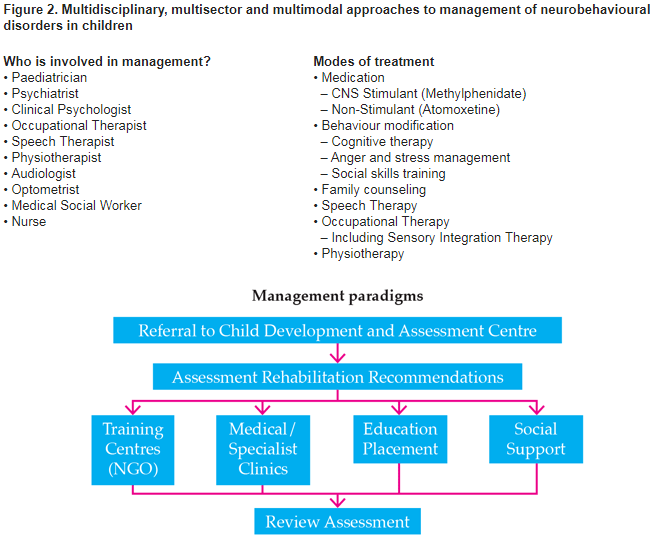
Multidisciplinary, multisector and multimodal approaches are required for the management of neurobehavioural disorders in children (Figure 2).
In addition to this multi-pronged approach, Dr Doo presented studies suggesting that symptoms related to ADHD and ASD may be ameliorated by omega-3 and/or omega -6 supplementation.
A randomized, placebo-controlled trial showed that after 6 months of daily omega-3 (558 mg EPA/174 mg DHA) and omega-6 (60 mg α-linoleic acid) treatment, 47% of children and adolescents (8–18 years old) with ADHD had improved scores on the ADHD Rating Scale-IV and Clinical Global Impression (CGI) scale.13 A systematic review revealed that although higher levels of essential fatty acids correlated with improved ADHD symptoms, such observations have been made in open-label, but not randomized clinical trials, suggesting that participants may be biased towards the benefits of supplementation.14 Further, a systematic review and meta-analysis showed that omega-3 fatty acid supplementation, particularly EPA induced a small but significant improvement in ADHD symptoms.15
A pilot, randomized controlled trial showed that administration of omega-3 fatty acids (1.3 g/day) consisting predominantly of DHA (230 mg/day) and EPA (350 mg/day) to ASD children aged 3–8 years for a total of 12 weeks may improve hyperactivity but the difference in change scores between the omega-3 and placebo groups did not show a statistically significant benefit on hyperactivity.16 A systematic review concluded that there is currently insufficient evidence to determine if omega-3 fatty acids are beneficial for ASD children.17
Until the advent of robust clinical trials showing the benefits of LC-PUFA supplementation, Dr Doo advised parents to follow the SECS (Safe, Easy, Cheap and Sensible) versus RUDE (Risky, Unrealistic, Difficult and Expensive) rule when deciding treatment options for neurobehavioural disorders. Although evidence of its benefits may not be highly conclusive, LC-PUFAs may be a sensible choice for use to help improve symptoms as it is safe, easy and cheap. Apart from LC-PUFA supplementation, children with neurobehavioural disorders should maintain a regular eating schedule. Nutrition counseling should be considered as a treatment option in the management of neurobehavioural disorders in children.
Conclusion
Currently, there is strong evidence that pre- and early post-natal DHA supplementation can have a significantly positive impact on the cognitive and vital functions of children, particularly later in childhood where tests for more specific cognitive functions can be performed. Although limited, there is evidence that DHA supplementation may ameliorate the symptoms of inattention and hyperactivity in children with ADHD and ASD. As it is safe, easy, cheap and sensible, health care providers may consider recommending DHA supplementation as a means for improving the symptoms of neurobehavioural disorders in children.
References
1. Agostoni C. J Pediatr Gastroenterol Nutr 2008; 47(Suppl 2):S41-4
2. Martinez M. J Pediatr 1992; 120(4 Pt 2): S129-38.
3. Colombo J et al. Pediatr Res 2011; 70(4): 406-10.
4. Colombo J et al. Child Dev 2004; 75(4): 1254-67.
5. Carlson SE et al. Am J Clin Nutr 2013; 97(4): 808-15.
6. Colombo J et al. Am J Clin Nutr 2013; 98(2): 403-12
7. Richardson AJ et al. PLoS One 2012; 7(9): e43909.
8. Reiersen AM and Todd RD. Expert Rev Neurother 2008; 8(4): 657-69
9. Polanczyk G et al. Am J Psychiatry 2007; 164(6): 942-8.
10. Leung PW et al. Eur Child Adolesc Psychiatry 2008; 17(7): 452-61.
11. Leung PW et al. Br J Psychiatry 1996; 168(4): 486-96.
12. Child Assessment Services Department of Health Hong Kong. Child Assessment Service Epidemiology and Research Bulletin (Issue 3). 2007.
13. Johnson M et al. J Atten Disord 2009; 12(5): 394-401.
14. Raz R and Gabis L. Dev Med Child Neurology 2009; 51(8): 580-92.
15. Bloch MH and Qawasmi A. J Am Acad Child Adolesc Psychiatry 2011; 50(10): 991-1000.
16. Bent S et al. J Autism Dev Disord 2011; 41(5): 545-54.
17. Bent S et al. J Autism Dev Disord 2009; 39(8): 1145-54.
